A common type of hydrolysis occurs when a salt of a weak acid or. 2KMnO 4 16 HCl 2KCl 2MnCl 2 5Cl 2 8H 2 O.
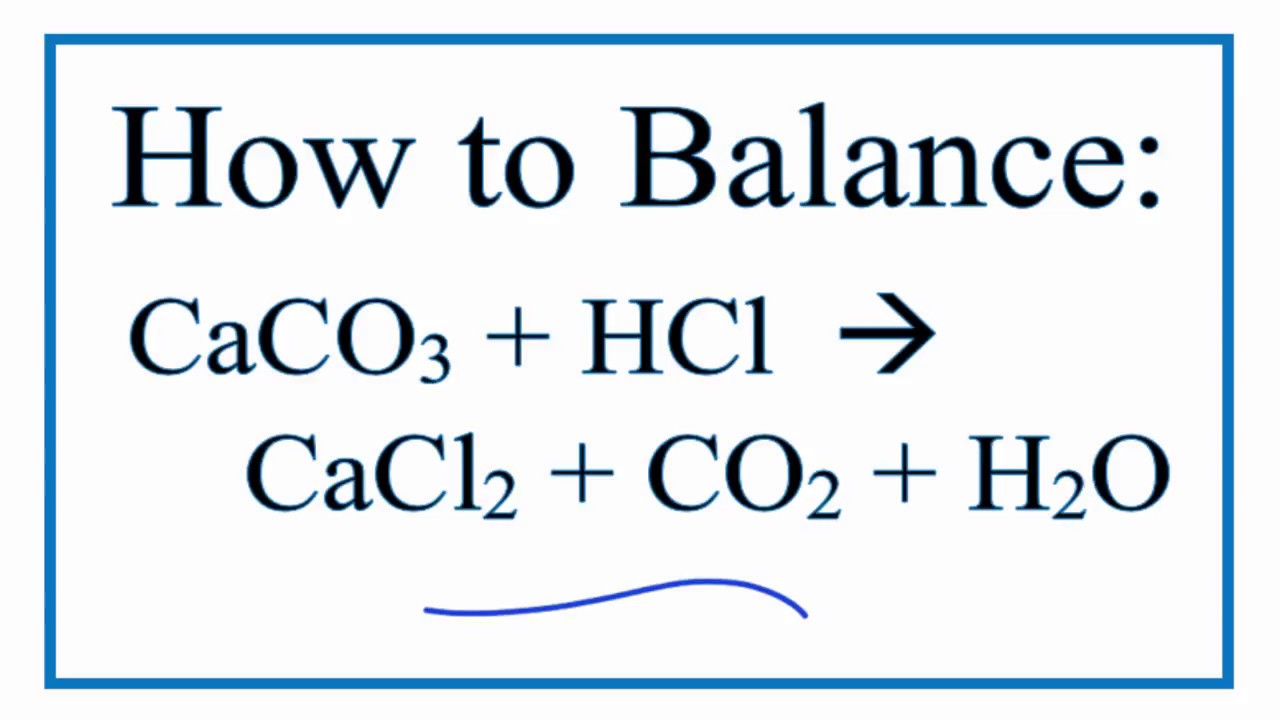
How To Balance Caco3 Hcl Cacl2 Co2 H2o Calcium Carbonate Hydrochloric Acid Youtube
Answer CaCO 3 s 2HCl aq CaCl 2 aq CO 2 g H 2 O l.
. Write a balanced chemical equation for the reaction if one of the compounds formed is. 53 Quick check 5 Construct i a balanced chemical equation and ii an ionic equation for each of the following reactions. Given the following balanced chemical equation how many grams of Hg would be produced from the A.
1 Sulphuric acid potassium hydroxide 2 Nitric acid sodium hydroxide 3 Silver nitrate solution sodium chloride solution 4 Calcium carbonate hydrochloric acid 5 Magnesium hydrochloric acid Solution Chapter 10 Acids Bases and. Therefore the reaction between calcium carbonate and hydrochloric acid is. Hydrochloric acid reacts with salts like carbonates hydrogen carbonates.
Phosphorous pentoxide and calcium oxide are good drying agents but they cannot be used to dry hydrogen chloride gas because they react with hydrogen chloride. The grey-silvery solid zinc dissolves in hydrochloric acid with effervescence to evolve hydrogen gas and leave a colourless solution of the salt zinc. Milk of magnesia chemically magnesium hydroxide is used as an antacid.
Percent yield represents the ratio between what is experimentally obtained and what is theoretically calculated multiplied by 100. As the end product is calcium chloride and the gas formed is carbon dioxide the metal compound A must be calcium carbonate. Metal acid a salt hydrogen The salt and its name depends on the metal and acid used in the reaction and the acid is neutralised in the process.
Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride. P ΔH ΔQ ΔV. Chemical Properties of Hydrochloric acid HCl.
C 2 H 6 O 2 2CO 2 3H 2 O. C a O H 2 H 3 P O 4 C a 3 P O 4 2 H 2 O unbalanced 3 C a O H 2 2 H 3 P O 4 C a 3 P O 4 2 6 H 2 O balanced equation Dibasic calcium phosphate can be produced in the former of these reactions by using an aqueous. HCl can be oxidized by potassium permanganate KMnO4 or potassium dichromate K2Cr2O7 liberated chlorine gas.
Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride. C 2 H 6 72 O 2 2CO 2 3H 2 O x 2. In chemistry acid hydrolysis is a process in which a protic acid is used to catalyze the cleavage of a chemical bond via a nucleophile substitution reaction with the addition of the elements of water H 2 O.
2K 2 Cr 2 O 7 14 HCl 2KCl 2CrCl 3 3Cl 2 7H 2 O. However an online Chemical Equation Balancer Calculator will provide you the balanced equation equilibrium constant with chemical name and formula of all reactants and product of a chemical equation. The gas evolved extinguishes a burning candle.
Speight in Reaction Mechanisms in Environmental Engineering 2018 31 Acid Hydrolysis. You want to measure how much water is produced when 120 g of glucose C_6H_12O_6 is burned with enough oxygen. The balanced equation for the reaction.
Aluminum and hydrochloric acid react to form aluminum. In Section 21 we have seen that all acids have similar chemical. Click hereto get an answer to your question Metal compound A reacts with dilute hydrochloric acid to produce effervescence.
A balancing chemical equation worksheet is a practice booklet with unsolved and solved chemical equation problems on which students. The gas evolved extinguishes a burning candle. Metal compound A reacts with dilute hydrochloric acid to produce effervescence.
This is a balanced chemical equation representing the reaction between sodium metal and hydrochloric acid reactants to produce sodium chloride solution and hydrogen gas products. Since it is basic in nature reacts with the excess hydrochloric acid present in the stomach and neutralises it. Chemistry - how to write balanced ionic equations Molecular Complete Ionic and Net Ionic Equations How to write ionic and net ionic equations How to write a double replacement net ionic equation what are spectator ions precipitation reaction single displacement reaction with video lessons examples and step-by-step solutions.
There are 7 O-atoms on RHS. Now to balance the atoms in carbon multiply CO 2 molecules by 2. What Is a Balancing Chemical Equations Worksheet.
How can I balance this chemical equations. 22 WHAT DO ALL ACIDS AND ALL BASES HAVE IN COMMON. The drying agent used in drying hydrogen chloride gas is conc.
The acidity produced due to excess hydrochloric acid in the stomach which cause indigestion produce pain and irritation. It can also be produced by reacting phosphoric acid H 3 PO 4 with solid calcium hydroxide CaOH 2. Write a balanced chemical equation for the reaction if one of the compounds formed is calcium chloride.
Mass of HgO 564 g We know that molar mass of HgO 21659 g Molar mass of Hg 20059 g. By making considerable change in enthalpy equation we get. To make 7 O-atoms at LHS we have to write 72 before O 2 but we can use only whole number to balance the equation so we write 72before O 2 and multiply the whole equation by 2.
Metal compound A reacts with dilute hydrochloric acid to produce effervescence. Concentrated H 2 SO 4. Yield actual yieldtheoretical yield 100 So lets say you want to do an experiment in the lab.
The gas evolved extinguishes a burning candle. P 7 41 11. REACTION OF ACIDS WITH METALS.

A Substitute Formulae For Names And Balance The Following Equation Calcium Carbonate Reacts Youtube

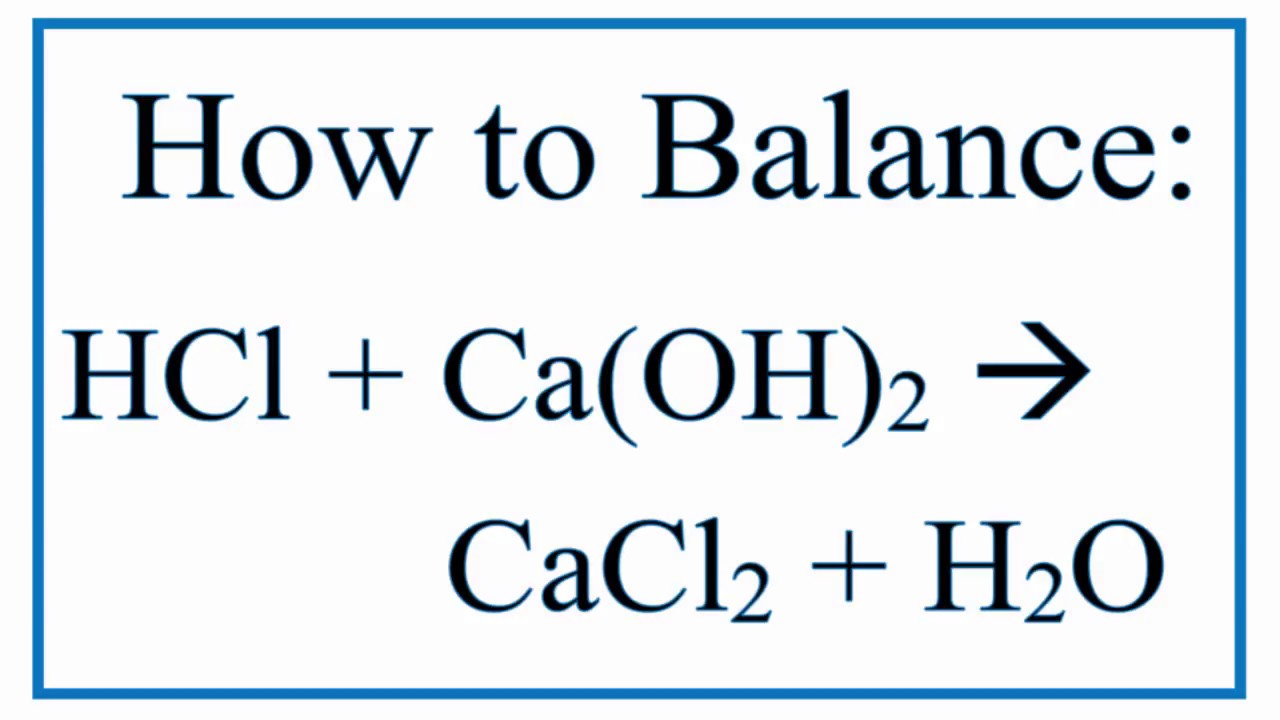
Balance Hcl Ca Oh 2 Cacl2 H2o Hydrochloric Acid And Calcium Hydroxide Youtube

How To Balance Ca Hcl Cacl2 H2 Calcium Hydrochloric Acid Youtube

How To Balance Ca Hcl Cacl2 H2 Calcium Hydrochloric Acid Youtube
0 Comments